Lung cancer is the most common cause of cancer-related deaths worldwide. More people die from lung cancer alone than from breast, prostate, colon and pancreas cancer combined. In Singapore. It is the most common cause of death in males, and second most common in females, lagging behind breast cancer by only 100 deaths a year. The main reason for the high death rate in lung cancer is the failure to detect it early.
CONVENTIONAL DIAGNOSIS OF LUNG CANCER
There are several ways by which lung cancer is detected. In most cases diagnosed in the early curable stage, the starting point is an abnormal chest x-ray or an abnormal CT scan of the chest that was done either to evaluate some chest symptom, or as part of a voluntary health screening, preemployment health check, pre-dependent pass health check or pre-visa application health check. In cases where the lung cancer is diagnosed in advanced stages, it is symptoms like cough, coughing up blood or shortness of breath that leads to the diagnosis.
Once an abnormality is detected on the x-ray or CT scan, the next step undertaken is to collect a sample (biopsy) from the abnormal area in the lungs. It used to be sufficient to just establish if the abnormal area is cancerous (or not) when treatment options were limited to chemotherapy or radiotherapy. However, this is no longer so.
The bar has been raised to identify the type of mutation driving the cancer as the treatment options vary by mutation, such as tablet therapy (targeted therapy) or immunotherapy. To achieve this, there must be an adequate quantity of tissue sample collected during the biopsy procedure and this must be done in a minimally invasive manner.
The task of confirming the cancer and finding its mutation-type rests on the biopsy. This is most commonly achieved either by passing the bronchoscope, with or without an ultrasound at its tip, through the mouth into the windpipe and lungs to collect the sample from the abnormal area, or it is done by passing the needle from the skin directly into the chest (see Figure 1).
However, the lung, being similar to an inflated balloon filled with air, carries a risk of being punctured by the latter mode of biopsy when the needle is being passed from the skin into the lungs. Hence, this modality is only suitable for cancers that are located toward the outer margin of the lungs as the needle has to travel less distance into the lung, lowering the risk of puncture.
Due to this risk, passing the bronchoscope with or without an ultrasound at its tip through the mouth into the windpipe for biopsy is considered a safer alternative as the bronchoscope can be advanced as close as possible to the abnormal area, while avoiding puncturing the lung (see Figure 2).
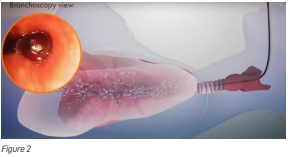
However, this method can be challenging as well because it entails three factors: reaching the right place for the biopsy; not slipping away from the right place while performing the biopsy; and minimising complications, such as bleeding and puncturing the lungs from the tools used for the biopsy.
Consequently, this technique has historically been limited by:
- Discrepancy between the diameter of the bronchoscope and the diameter of the windpipes;
- Difficulty in ensuring the biopsy is being done from the most abnormal area rather than the less abnormal area to avoid false negative result;
- Inability to bend the bronchoscope sufficiently in keeping with the branching pattern of the wind pipes; and
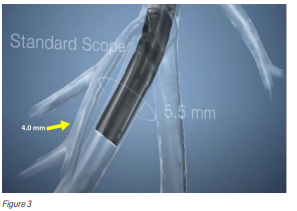
- Difficulty visualising the biopsy tools and their position deep within the lungs while the biopsy is being carried out (see Figure 3).
OVERCOMING DISCREPANCY BETWEEN THE DIAMETER OF THE BRONCHOSCOPE AND THE DIAMETER OF THE WIND PIPES
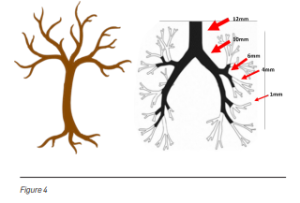
Windpipes resemble an upside-down tree. The branches of the tree get smaller as they stretch away from the trunk. Similarly, the branches of the windpipes in the lungs get smaller in diameter as they branch away from the centre of the lungs to the periphery (see Figure 4). The bronchoscopy approach, whereby a camera and a light are passed down the windpipes into the lungs, encounters difficulty as it can only go until where the windpipes’ diameter is larger than the diameter of the scope. The scope cannot advance when its diameter is larger than the diameter of the airways. Due to this, it becomes difficult to reach the peripheral areas of the lung to perform biopsy when the cancer is located there.
ULTRATHIN 3.00mm BRONCHOSCOPE
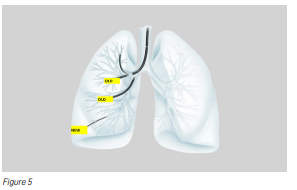
Recently, new advancements in technology have enabled doctors to overcome this limitation by using an ultrathin hybrid bronchoscope, whose distal end has an outer diameter of only 3.0mm (size of a black peppercorn). The narrower scope diameter of 3.0mm makes it possible to reach the peripheral bronchi that could not be reached earlier (see Figure 5).
ENSURING BIOPSY IS DONE FROM THE MOST ABNORMAL AREA TO AVOID FALSE NEGATIVE RESULT
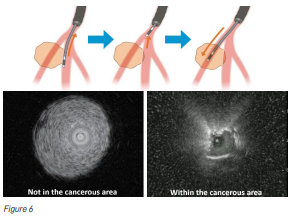
The other issue faced by doctors is that while doing the biopsy, they may find it difficult to ascertain whether they are doing the biopsy within the centre of the lesion where they will be able to get sufficient tissue for making the diagnosis, or whether they are doing the biopsy at the edges of the abnormal area where they may miss the diagnosis (see Figure 6). The solution to this problem was to pass an ultrasound (US) probe through the bronchoscope. However, the diameter of such US probes tended to limit their reach. Recently, like the bronchoscope, the diameter of the US probes has been further reduced to allow them to reach where they could not reach before. The thin radial type US probe allows visualisation of the peripheral pulmonary lesion, checking of the lesion position, and performing of sampling with the radial US probe.

INABILITY TO BEND THE BRONCHOSCOPE SUFFICIENTLY
The mainland of Singapore measures 50km from east to west and 27km from north to south, with 193 kilometres of coastline. Our two lungs together contain approximately 2,400km of windpipes within them. This long length is folded and squeezed into our small chest; due to which, the windpipes have curvatures and bends. This means the biopsy tools must be flexible enough to bend along the bends in the wind pipes.
MINIATURISATION OF THE CONVEX ULTRASONIC PROBE – NEW SLIM EBUS BRONCHOSCOPE
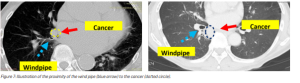
While the radial probe EBUS is used for the peripherally located cancers, the convex probe endobronchial ultrasound (EBUS) is used for the more centrally located cancers. However, the diameter of the current convex probe EBUS is large (6.9 mm) and its flexibility is limited. When the cancer is located next to windpipes that are smaller than 6.9 mm in diameter or at an angle to which the current EBUS scope cannot be bent, the collection of tissue (biopsy) becomes difficult (see Figure 7).
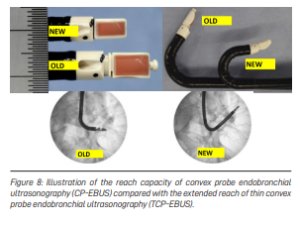
This limitation has been overcome by the development of a smaller convex probe EBUS bronchoscope that can reach further away into the lungs and can bend more, compared to the currently available scope (see Figure 8).
VISUALISING THE BIOPSY TOOLS AND THEIR POSITION DEEP WITHIN THE LUNGS WHILE CARRYING OUT BIOPSY
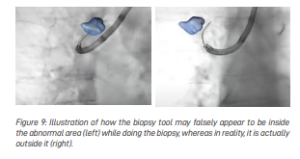
Another difficulty that doctors encounter when x-ray is used to watch the biopsy tools during the biopsy procedure (to ensure that the tools are targeted in the right place and the biopsy is indeed taking place at the area of abnormality and not the normal lung) is that the x-rays only provide a two-dimensional view. Hence, it can be difficult to tell if the biopsy tool is within the abnormal area, in front of it or behind it because on a two-dimensional plane, it is hard to see the difference (see Figure 9).
AUGMENTED FLUOROSCOPY FOR NAVIGATION
This technology combines the two-dimensional x-ray pictures of the lungs during the biopsy procedure with the CT scan that was done earlier, and creates a three-dimensional structural road map of the lungs, generating a potential pathway for the nodule like a GPS system creating a road map to reach any destination.
CONCLUSION
Advancements in lung cancer treatment are progressing at a rapid pace. The standard of care in 2020 for early-stage lung cancer used to be its removal by surgery alone, conferring 5-year survival in 65% of patients. However, the standard of care in the same group of patients now is additional targeted therapy after the surgery, conferring 5-year survival in 85% of patients. It is important for the diagnostic modalities to keep up with the pace of therapeutic advancements. And thanks to the above discussed technologies, we are achieving this goal.
Dr Akash Verma
Lung Specialist & Interventional Pulmonologist
Mount Elizabeth Hospital
MBBS, MRCP (UK), FAMS, FRCP (Edin)
Dr Akash Verma is a Lung specialist & Interventional Pulmonologist practising at Mount Elizabeth Hospital. He sub-specialises in Intensive Care Medicine & Advanced Bronchoscopy, and performs both flexible and rigid bronchoscopy with techniques of laser resection of lung cancer, silicone airway stenting, pleuroscopy and navigation bronchoscopy. Prior to joining Mount Elizabeth Hospital, Dr Akash was a Senior Consultant in Respiratory Medicine at TTSH. After graduation, he trained at TTSH before obtaining his membership of Royal College of Physicians (UK). Upon completion of advanced training in Respiratory Medicine and Critical Care, He was conferred a Fellow of the Academy of Medicine, Singapore (FAMS). Subsequently, he was awarded the Health Manpower Development Program (HMDP) fellowship and was a visiting fellow in the department of Interventional Pulmonology at Samsung Medical CentreSeoul, Mayo Clinic-USA, and University of Chicago.













