By Dr Akash Verma
Lung cancer is the leading cause of cancer-related deaths worldwide. More people die from lung cancer alone than from breast, prostate, colon and pancreatic cancers combined. In Singapore, it is the most common cancer-related cause of death in men and the second most common in women, trailing breast cancer by only about 100 deaths a year. The main reason for this high mortality rate is the difficulty in detecting lung cancer early.
Conventional Methods of Diagnosing Lung Cancer
Doctors detect lung cancer in several ways. Many early-stage and curable cases start with an abnormal chest X-ray or CT scan done for symptoms such as cough or chest pain, or as part of routine checks—health screening, pre-employment assessments, or health checks before work passes and visas. Advanced cancers, on the other hand, usually come to light when patients develop symptoms such as coughing, coughing up blood, or shortness of breath.
Once a scan shows an abnormality, the next step is to obtain a tissue sample (biopsy). In the past, confirming whether the abnormality was cancerous was enough because treatment options were limited to chemotherapy or radiotherapy. Today, doctors need to identify the specific genetic mutation driving the cancer, as treatment choices—such as targeted tablet therapy or immunotherapy—depend on it. This makes it essential to collect an adequate tissue sample through minimally invasive methods.
Doctors most commonly perform the biopsy by passing a bronchoscope—sometimes with an ultrasound at its tip—through the mouth into the windpipe and lungs. Alternatively, they insert a needle from the skin directly into the lung (Figure 1). However, because the lung behaves like an inflated balloon filled with air, inserting a needle through the chest wall carries a risk of puncture. This method is therefore safer when the abnormality lies near the outer part of the lung.

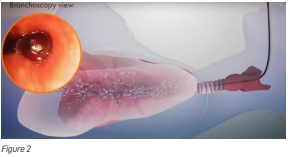
Bronchoscopy reduces this risk because the scope can be positioned close to the abnormal area without puncturing lung tissue (Figure 2). However, bronchoscopy brings several challenges:
-
reaching the correct location,
-
staying in the correct location during sampling, and
-
reducing complications such as bleeding or accidental puncture from biopsy tools.
Historically, bronchoscopy-based biopsies have been limited by:
-
A mismatch between the bronchoscope diameter and the narrowing airway branches.
-
Difficulty ensuring the biopsy targets the most abnormal portion of the lesion to avoid false negatives.
-
Limited ability to bend the bronchoscope in line with the natural branching patterns of the airway.
-
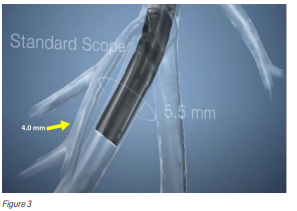
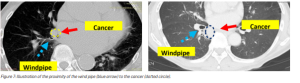
Poor visibility of biopsy tools when working deep inside the lungs (Figure 3).
Overcoming the Diameter Mismatch Problem
The airway branches resemble an upside-down tree. As the bronchi extend from the centre of the lung towards the outer regions, their diameter narrows (Figure 4). Traditional bronchoscopes cannot advance once the airway becomes smaller than the scope itself. This makes it difficult to reach abnormalities located in the peripheral lung regions.
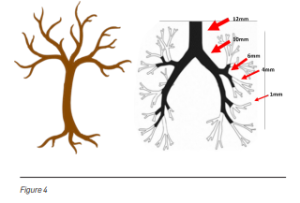
Ultrathin 3.0 mm Bronchoscope
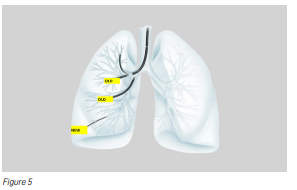
Recent technological advances have produced an ultrathin hybrid bronchoscope with a distal end diameter of only 3.0 mm—about the size of a black peppercorn. This allows doctors to reach peripheral bronchi that were previously inaccessible (Figure 5).
Ensuring Biopsies Target the Most Abnormal Area
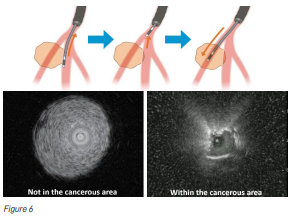
Another major challenge is confirming that the biopsy tool is sampling the most abnormal part of the lesion, not its edges (Figure 6). Sampling the margins may produce insufficient tissue or even miss the cancer entirely. To address this, doctors insert an ultrasound (US) probe through the bronchoscope. Older probes were too wide to reach deep lesions, but newer, thinner radial US probes now allow doctors to visualise peripheral lesions, confirm their position, and take targeted samples.

Improving Flexibility to Navigate Bends in the Lungs
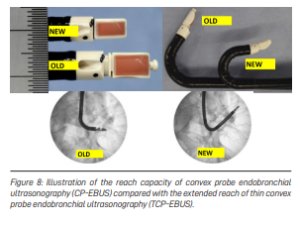
Although Singapore is small, our lungs contain about 2,400 km of airways—folded and curved inside the chest. Biopsy tools must bend along these sharp angles. Traditional convex probe endobronchial ultrasound (EBUS) scopes measure 6.9 mm in diameter and have limited flexibility. When a tumour lies next to a small airway or at a tight angle, taking a sample becomes very difficult (Figure 7).
Slimmer Convex Probe EBUS Bronchoscope
A newer, slimmer convex probe EBUS scope now addresses this limitation. It can reach further into the lungs and bend more effectively than current scopes, enabling easier access to centrally located abnormalities (Figure 8).
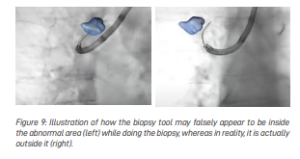
Improving Visualisation of Tools Deep Within the Lungs
When doctors rely on X-rays to guide biopsy tools, they see only a two-dimensional image. This makes it hard to tell whether the tool is inside, in front of, or behind the abnormal area (Figure 9).
Augmented Fluoroscopy for Better Navigation
Augmented fluoroscopy merges real-time X-ray images with the patient’s earlier CT scan. The system creates a three-dimensional “road map” of the lungs, similar to a GPS guiding a driver to a destination. This helps doctors navigate precisely to the lesion and sample the correct spot.
Conclusion
Lung cancer treatment has advanced rapidly. In 2020, early-stage lung cancer treatment usually involved surgery alone, with a five-year survival rate of about 65%. Today, patients receive additional targeted therapy after surgery, improving five-year survival rates to about 85%. Diagnostic techniques must evolve alongside these treatment advances. Thanks to the technologies discussed above, doctors are getting closer to earlier, safer, and more accurate detection of lung cancer. PRIME












Leave A Comment